We went through and did quite a number of worksheets today. The worksheets had let us realise where our mistakes were and where we had went wrong.
3 Thoughts:
1) When we place the balloon in the freezer for some time, it will become smaller in size. This is because when temperature decreases, the particles in the balloon will lose energy and move less quickly. They will then become closer and the air contracts. Thus, causing the balloon to become smaller.
2) Ice has a higher density than water vapour as ice, which is in the solid state, has particles that are arranged closely together. There are more particles packed in ice (solid) than in the same volume of water vapour (gas). Therefore, ice has a greater mass than water vapour in a fixed volume, hence a higher density.
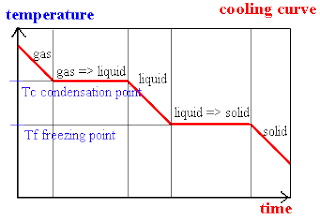
3) Impurities do affect the melting and boiling points of a substance. Impurities will lower the melting point of a solid and increase the boiling point of a liquid and thus, causing the substance to melt and boil respectively over a range of temperatures.
2 Questions:
1) When we place the balloon in the freezer, will the air inside comes to a point where it changes into liquid or solid states?
2) Do the particles in matter have a fixed mass?
1 Useful application:
1) After we had found out our mistakes, we will ensure not to answer the questions in our worksheets or test papers wrongly again. We always learn from mistakes! :)
(Salt - One Common Type of Impurities)
Sources: Google Images