I used to think that for heating and cooling curves, the temperatures would continuously rise and fall respectively. But now, after gaining the knowledge of these two types of curves, I think that the temperatures would remain constant at some points when the matter is changing into another state.
For heating curves, temperature remains the same for a period of time so that heat supplied is used to overcome the forces of attraction holding the particles together. The temperature will then rise when the matter has completely changed into another state.
Note: Avoid using BREAK. Instead, use OVERCOME.
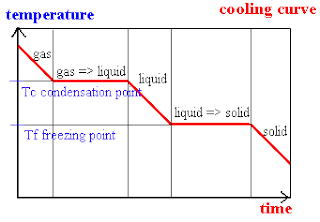
As for cooling curves, temperature stays constant for some time as heat energy is released so that strong attractions are formed between particles to hold them together in a crystal lattice (solid structure). Then, the temperature will continue to fall when the matter has completely changed into another state.
Sources: Google Images

No comments:
Post a Comment